Successful transport relies on a combination of the right equipment, proven safety protocols, and the expertise of clinicians.
By Dillon Stickle
![neonatal-icu-nicu-hospital-500]()
Transferring critically-ill neonatal and pediatric patients for diagnostic or therapeutic purposes can prove to be a challenge for healthcare facilities in terms of proper care and safety of the patients. The success of NICU/PICU transports relies heavily on proven safety protocols, proper ventilation and airway management equipment and technology, and, most importantly, the expertise of clinicians like respiratory therapists (RT). Without these important factors, the patients could be at risk of serious and sometimes fatal adverse events.
The Role of the RT
Respiratory therapists play a critical role during transport of neonatal and pediatric patients. They offer expertise in treatments for respiratory medicine, cardiopulmonary pathophysiology, and the employment of proper technology and equipment to provide optimum care for young, critically ill patients who are in need of an inter-facility transport (IFT).
“The vast majority of specialized neonatal and pediatric transport teams are staffed by an RN and an RRT,” said Steve Sittig, RRT-NPS, CNPT, FAARC. “The use of RTs in medical transport began in the 1970s when specialized neonatal transport was developed. In the 1980s, more institutions began to develop specialized pediatric transport teams of which respiratory therapists were considered an integral team member.”
Now, he said, almost all progressive neonatal and pediatric specialty transport teams utilize the transport RT for many advanced procedures such as intubation, peripheral IVs, arterial line placement, point of care blood analysis, and chest tubes for pneumothorax. “These advanced skills can vary from institution to institution,” he added.
Sittig also explained that many teams are now requiring transport RTs to hold the RRT credential, as well as the neonatal pediatric specialty (NPS) credential or the certification of neonatal pediatric transport (CNPT).
“For transport teams who are certified by the Commission on Accreditation of Medical Transport Services (CAMTS), these advanced credentials will be required by 2019 when the 11th Edition of the CAMTS Standards are published,” he said. “These advanced exams show that the transport RT understands the unique environment of transport along with specific pathophysiologies typically present in this population requiring medical transport.”
Best Practices and Proper Protocols
In a 2012 study,1 researchers investigated the risk factors associated to clinical deterioration in neonatal patients during IFT. The research team looked at the Transport Risk Index of Physiology Stability (TRIPS) risk score—which has shown efficacy2 in predicting early mortality at admission—in 160 newborn infants pre- and post-transport. A diagnosis of clinical deterioration was made if the score was higher post-transport. The results showed a staggering 91 (57%) neonatal patients suffered clinical deterioration during IFT; 49 of those patients required immediate cardiorespiratory support (ICRS) and 28 died post-transport.
This study concluded that it is crucial for transport teams to optimize care strategies in order to deter the deterioration of young, vulnerable patients during IFTs, which will in turn reduce the number of patients who require ICRS as well as lower mortality rates.
It goes without saying there are significant risks to transporting critically-ill neonatal and pediatric patients. From failed intubations to overcooling to safety for both patients and clinicians, the transport team must be prepared to address these risks using proven best practices and up-to-date protocols.
Bradley Kuch MHA, RRT-NPS, FAARC, director of respiratory care services and transport team at the Children’s Hospital of Pittsburgh of UPMC, describes some of the things he and his team have in place that have been shown to improve outcomes.
“One practice we use is active cooling, which has been shown to be very successful and produce good outcomes. Most teams right now are doing passive cooling, but we believe actively controlling temperatures is definitely the best way to go,” he said.
Research backs up Kuch’s assessment of active versus passive cooling. In one study, published in the journal Pediatrics,3 researchers set out to compare outcomes between passive and active cooling. Of the 134 patients transported, 64 were passively cooled—and only 39% of the patients were within the target temperature range when arriving to the receiving facility. In contrast, an impressive 100% of patients that underwent active cooling were within the target temperature range. Active cooling was shown to improve temperature stability, decrease the incidence of overcooling, and reduce stabilization and transfer time, according to the authors of the study.
A good response time by the transport team is also crucial when it comes to neonatal patients, especially during the first hour of a patient’s life, also known as the golden hour. “In neonatal and pediatric critical care transports,” said Kuch, “the golden hour for us is getting that highly specialized team to the hospital to prepare the patient, and initiating proper communication with the outside facility.” An integral part of the team’s communication system are their specialized command positions: one for the NICU who is a neonatologist, and one for the PICU who is a pediatric intensivist. “They act as the point person for hospital transports and play a big role in getting the teams ready as quickly as possible, and facilitating communication between facilities,” he says.
“One of the biggest things I believe helps our patients and our team in airway management is our use of the video guided intubations,” said Olivia Kaullen, RRT-NPS. She says she not only uses this technology to help herself intubate better, but also for education and for allowing other team members to help guide the intubation if necessary. “It is our protocol to use this device first every time we intubate. Our first-attempt intubation rate has actually improved which may be due to this policy.”
Kuch added that it is important to always keep in mind the safety of the patient and crew. “The assessments need to be looking at having the right equipment to protect them; protecting the patient from loud noises, making sure vehicles are operated safely and effectively, making sure the patient is secured at all times, and so on.”
A major factor when it comes to safety is during inclement weather. “If for any reason they team feels that the weather of patient condition is not conducive to safe transport, most transport team use the phrase ‘All to go, one to say no,’” said Sittig. “So, if any one team member is not comfortable with the weather conditions, the transport may be delayed until weather improves. If you look at the history of medical transport accidents, most occur when programs push the weather in order to complete a transport.”
Improving Outcomes with Innovative Equipment
“The advent of new technology has vastly improved quality of care in transport,” said Sittig. “New transport ventilators can rival hospital ICU vents for adaptive modes of ventilation, and transport RTs can now bring these new modes of ventilation to the patient wherever they are located.”
One of these modes of ventilation is the Hamilton-T1, created by Hamilton Medical and designed specifically for fragile neonates, which performs on the same level as an NICU bedside ventilator. This product features an nCPAP-PC (pressure control) mode in which the RT defines the desired CPAP target value of a patient and the ventilator adapts the required flow to the patient’s condition. In turn, the patient receives only as much flow as is necessary to reach the target value. According to the company’s press release, this reduces work of breathing and the need for user interventions, and also ensures optimal leak compensation.
NeoTech Products also has a couple products on the market designed to make IFTs easier. The NeoBar ET Tube Holder is designed to reduce extubations, help prevent palate trauma and allows for better oral care. It also eliminates the need to tape near the patient’s nose or mouth. Another product they offer is the NeoGrip Tubing and Cable Holder, which helps to keep tubes, cables and circuits together and allows for better patient mobility.
Kaullen warns that while there is a lot of technology that transport teams can use to better care for patients, none of it can reach its full potential if the team is not trained to use it appropriately. “It is very important that the personnel using the respiratory equipment not only understand how to use it appropriately, but also how it works and how to troubleshoot it,” she said. “When you’re out in the field, you can’t have another RT come help you—you must be knowledgeable for the sake of your patient.”
The Necessity of Experience and Communication
In a retrospective study4 aimed at evaluating and identifying potential complications during IFT of critically-ill pediatric patients, researchers found that the most significant obstacle faced during the process was a lack of knowledge or experience of the transport team to perform safe, effective transfers. The abstract, published in the Journal of the Medical Association of Thailand, concludes that in order to improve the outcomes and avoid the potential adverse events during IFT, it is imperative that the transport team be organized and prepared with proper safety protocols.
Kuch believes that a well-experienced and prepared team of clinicians is the key to successful transfers. “Both our nurses and therapists, as well as other physicians, are required to have at least three years of neonatal and/or pediatric experience, because that is the best way to ensure that we reach the best outcomes for the patient,” he said.
In a single-center, prospective, cohort study5 published in the journal Pediatrics, researchers hypothesized whether a specialized pediatric team, compared to a non-specialized team, would improve rate of survival as well as lower unplanned, adverse events during transport. The study concluded that the non-specialized team was associated with a higher frequency of unplanned, adverse events, as well as a higher rate of mortality among pediatric patients. This shows that having a well-rounded, specialized critical care team is associated with better outcomes for patients during IFT.
“I think communication and collaboration is another key to a successful and reliable transport team,” said Jennifer Mahone, MHA, RRT-NPS, neonatal/pediatric clinical specialist at Reading Hospital. “Truly making sure that everything is in place to keep the patients safe by working and communicating with the whole transport team is important for any hospital that provides these services.”
While good communication within the specialized team is a vital component to successful transfers, Kuch says it is also important to note the significance of proper communication and collaboration with outside facilities who are referring or receiving the patient. “We are in what I would call a ‘resource-limited environment’ and it is really important that everyone on our end works with outside resources to help things get done the right way,” said Kuch. “I’d be remiss to not point out that good partnerships with those referring hospitals is key. It’s important that both the referring and receiving hospital provide good recommendations and different input for the patients. You need to have a good relationship with the outside hospital because, at the end of the day, both systems have the same goal in mind—providing optimal care for the patient.” RT
Dillon Stickle is associate editor of RT. For more information, contact editor@nullRTmagazine.com.
References
- https://www.ncbi.nlm.nih.gov/pubmed/22859323
- https://www.ncbi.nlm.nih.gov/pubmed/22495897
- http://pediatrics.aappublications.org/content/132/5/841.full
- https://www.ncbi.nlm.nih.gov/pubmed/16858849
- https://www.ncbi.nlm.nih.gov/pubmed/19564281
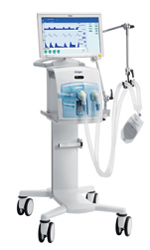 Dräger’s Evita Infinity V500 critical care ventilator is suitable for all patient ranges: neonatal, pediatric, and adult. It offers new concepts in ventilation, such as APRV with auto-release, configurable SmartCare/PS, and variable PS. It’s standardized nomenclature and customizable interface improves workflow and enhances ICU safety. The V500’s ability to provide invasive, noninvasive, and O2 therapy offers a comprehensive array of therapy to adapt to the patient’s changing requirements. 800-437-2437;
Dräger’s Evita Infinity V500 critical care ventilator is suitable for all patient ranges: neonatal, pediatric, and adult. It offers new concepts in ventilation, such as APRV with auto-release, configurable SmartCare/PS, and variable PS. It’s standardized nomenclature and customizable interface improves workflow and enhances ICU safety. The V500’s ability to provide invasive, noninvasive, and O2 therapy offers a comprehensive array of therapy to adapt to the patient’s changing requirements. 800-437-2437;  Now available from Ventec Life Systems, VOCSN integrates five separate devices, including a critical care ventilator, 6 LPM equivalent oxygen concentrator, Touch Button Cough assist, hospital grade Suction, and high performance Nebulizer, into one unified respiratory system. The VOCSN critical care ventilator provides invasive, noninvasive, and mouthpiece ventilation. Designed to work in hospital, institutional, transport, and home environments, VOCSN delivers a comprehensive set of ventilation modes and settings to meet patient’s needs. The advanced unified respiratory system combines responsive leak and circuit compensation as well as precision flow trigger controls to enable comfortable breathing and accurate therapy. 844-MY-VOCSN (844-698-6276);
Now available from Ventec Life Systems, VOCSN integrates five separate devices, including a critical care ventilator, 6 LPM equivalent oxygen concentrator, Touch Button Cough assist, hospital grade Suction, and high performance Nebulizer, into one unified respiratory system. The VOCSN critical care ventilator provides invasive, noninvasive, and mouthpiece ventilation. Designed to work in hospital, institutional, transport, and home environments, VOCSN delivers a comprehensive set of ventilation modes and settings to meet patient’s needs. The advanced unified respiratory system combines responsive leak and circuit compensation as well as precision flow trigger controls to enable comfortable breathing and accurate therapy. 844-MY-VOCSN (844-698-6276);  The Babylog VN500 from Dräger is a ventilation system designed to meet the unique challenges of the neonatal patient. The Babylog VN500 offers a wide array of neonatal features to minimize lung injury, improve safety, and improve the environment of care for infants to thrive. Accurate tidal volume delivery as low as 2cm3, measurement and compensation for ET tube leakage, and volume guarantee options ensure proper ventilation and monitoring of the smallest of patients. In addition to conventional ventilation therapies, the Babylog VN500 can provide non-invasive ventilation and O2 therapy which can greatly
The Babylog VN500 from Dräger is a ventilation system designed to meet the unique challenges of the neonatal patient. The Babylog VN500 offers a wide array of neonatal features to minimize lung injury, improve safety, and improve the environment of care for infants to thrive. Accurate tidal volume delivery as low as 2cm3, measurement and compensation for ET tube leakage, and volume guarantee options ensure proper ventilation and monitoring of the smallest of patients. In addition to conventional ventilation therapies, the Babylog VN500 can provide non-invasive ventilation and O2 therapy which can greatly The Servo-U, from Maquet, is designed to making protective ventilation more accessible, understandable, and easy to implement. The device can be used on neonates through adults and has a completely touch-based interface that helps clinicians manage ventilation. The Servo-U is equipped with NAVA (Neurally Adjusted Ventilatory Assist) technology and Edi (electrical diaphragmatic) monitoring for enhanced patient-ventilator interaction and greater insight into patient respiratory condition. The platform is designed to grow with the customer and can be upgraded easily and cost-effectively. 888-627-8383;
The Servo-U, from Maquet, is designed to making protective ventilation more accessible, understandable, and easy to implement. The device can be used on neonates through adults and has a completely touch-based interface that helps clinicians manage ventilation. The Servo-U is equipped with NAVA (Neurally Adjusted Ventilatory Assist) technology and Edi (electrical diaphragmatic) monitoring for enhanced patient-ventilator interaction and greater insight into patient respiratory condition. The platform is designed to grow with the customer and can be upgraded easily and cost-effectively. 888-627-8383;  Unlike conventional devices that require a tube to connect from a power unit, this device fits directly on the mask, completely eliminating dead space. This allows for lower pressures to be used, which the company claims will mean significantly increased comfort for the patient. The device can also be used with a tube, be clothes mounted, or put in a pocket.
Unlike conventional devices that require a tube to connect from a power unit, this device fits directly on the mask, completely eliminating dead space. This allows for lower pressures to be used, which the company claims will mean significantly increased comfort for the patient. The device can also be used with a tube, be clothes mounted, or put in a pocket.
 Gary Nieman, BS, MS
Gary Nieman, BS, MS Ron Pasewald BS, RRT-ACCS
Ron Pasewald BS, RRT-ACCS Daniel D. Rowley, MSc, RRT-ACCS, RRT-NPS, RPFT, FAARC
Daniel D. Rowley, MSc, RRT-ACCS, RRT-NPS, RPFT, FAARC

 The Philips Respironics V60 ventilator offers ICU-grade NIV performance with enhanced monitoring and alarms. The V60 uses auto-adaptive technology to help provide patient synchrony and therapy acceptance. With a six-hour battery, it is flexible enough for intra-hospital transport and offers treatment for a wide range of clinical severities for patients from pediatric to adult. 800-345-6443;
The Philips Respironics V60 ventilator offers ICU-grade NIV performance with enhanced monitoring and alarms. The V60 uses auto-adaptive technology to help provide patient synchrony and therapy acceptance. With a six-hour battery, it is flexible enough for intra-hospital transport and offers treatment for a wide range of clinical severities for patients from pediatric to adult. 800-345-6443; 

 Hayek Medical offers the Biphasic Cuirass Ventilation (BCV), which provides complete, noninvasive ventilation by replicating our natural breathing cycle through a cuirass shell. BCV is an alternative to traditional forms of ventilation. BCV also has secretion clearance (HFCWO) and a cough option. BCV can be used both in the hospital setting or at home. 855-243-8228;
Hayek Medical offers the Biphasic Cuirass Ventilation (BCV), which provides complete, noninvasive ventilation by replicating our natural breathing cycle through a cuirass shell. BCV is an alternative to traditional forms of ventilation. BCV also has secretion clearance (HFCWO) and a cough option. BCV can be used both in the hospital setting or at home. 855-243-8228; 